Correction of Postischemic Changes in the Microcirculation of Rat Cerebral Cortex with Mesenchymal Stem Cells
I. B. Sokolovaa,@, O. P. Gorshkovaa, and N. N. Pavlichenkob
aPavlov Institute of Physiology, Russian Academy of Sciences, St. Peterburg, 19903 Russia
b LLC Trans-Technology, St. Petersburg, 192146 Russia
*e-mail: SokolovaIB@infran.ru
Received May 28, 2021;
revised June 30, 2021;
accepted July 1, 2021
Abstract---This study aims at examining how intravenous transplantation of human mesenchymal stem cells (MSCs) performed on the day of ischemia/reperfusion affects the vascular density and the reactivity of the pial arteries and tissue perfusion in the cerebral cortex 7, 14, and 21 days after ischemic exposure. The density of the entire microvascular network and arterial vessels in the pial membrane of the sensorimotor cortex of rats undergoing cerebral ischemia/reperfusion (I/P) and MSC intravenous transplantation were assessed with equipment for microcirculation monitoring. The same setup was used to study the reactivity of the pial arteries exposed to acetylcholine (ACh). In parallel, the perfusion index (PI) was measured in the sensorimotor cortex using a LAKK-M laser Doppler system. The density of the entire microvascular network and arterial vessels decreased more than in the case of sham-operated (SO) rats in the first 7 days after I/R: by 1.6 and 1.4 times on average, respectively. After 14 days, these indicators were 1.4 and 1.2 times; after 21 days, 1.2 and 1.3 times. In animals that underwent I/R, the reactivity of the pial arteries to ACh significantly declined. Seven days after I/R, the number of dilating arteries decreased by 1.4--1.7 times; after 14 days, by 1.6--1.9 times; and, after 21 days, by 1.2--1.7 times. After 21 days, the PI level decreased statistically significantly (on average, by 1.6 times). Intravenous administration of MSCs preserved the density of the microvascular network of the pial membrane in rats at the level of control animals at all periods after I/R. The PI 21 days after I/R was 1.2 times lower than in the SO group, but statistically higher than in rats with ischemic brain injury without MSC administration. It is concluded that intravenous transplantation of MSCs made it possible to prevent degradation of the microvascular bed in the cerebral cortex of rats after I/R and to preserve the reactivity of the pial arteries at the level of control animals. The reactivity in the cell therapy group also did not differ from the control values. The PI 21 days after I/R was 1.2 times lower than in the SO group, but statistically higher than in rats with ischemic brain injury without MSC administration. It is concluded that intravenous transplantation of MSCs made it possible to prevent degradation of the microvascular system in the cerebral cortex of rats after I/R and to preserve the reactivity of the pial arteries at the level of control animals.
Keywords: ischemia/reperfusion, brain, intravenous transplantation, mesenchymal stem cells, microvascular density, reactivity, perfusion.
Abbreviations: BP---blood pressure, SO---sham-operated, MSC---mesenchymal stem cell, hMSC---human MS, PI---perfusion index, I/R---ischemia/reperfusion, TIA---transient ischemic attack, ED---endothelial dysfunction, ACh---acetylcholine.
INTRODUCTION
Disturbances of cerebral circulation resulting from the formation of a blood clot or prolonged spasm of cerebral arteries are one of the most common pathological conditions in modern everyday life (Shlyakhto et al., 2012). In about 30% of cases, the development of a stroke is preceded by short-term transient disorders of cerebral circulation, so-called “transient ischemic attacks” (TIAs), which do not lead to the development of a heart attack. Some of the main factors determining the outcome and long-term consequences of TIAs are the state of cerebral hemodynamics, the degree of its impairment, and the severity of compensatory mechanisms. The occurrence of repeated ischemic brain lesions is largely associated with micro- and macroangiopathies, leading to the development of hemodynamic disorders, based on endothelial dysfunction (ED). To date, it has been proven that endothelial cells are involved in many processes of regulation of hemostasis and microcirculation (Manukhina, Malyshev, 2003, Gimbrone, Garcia-Cardena, 2016). The development of ED results in an imbalanced production of substances that cause vasodilation and vasoconstriction and enhances the proliferation of smooth-muscle cells, adhesion, and thrombus formation. The walls of the cerebral arteries lose elasticity and thicken. The vascular lumen decreases, which can cause a deterioration in oxygen supply to brain tissue.
The development of methods to restore cerebral microcirculation and functions of the endothelium of cerebral vessels in various pathologies is an important task of modern medicine and biology. The application of mesenchymal stem cells (MSCs) for this purpose is an innovative and very promising approach. Numerous works over the past 30 years have shown that transplantation of MSCs after ischemic or traumatic brain injuries decreases the volume of damaged tissue, protects the neuronal function in the border zone of the damaged area, and restores microcirculation (Feng et al., 2016; Fitzsimmons et al., 2018). Administration of MSCs to restore the function of the endothelium of cerebral vessels after a TIA is a practically unexplored way of correcting postischemic complications. To date, in vitro and in vivo studies have shown that MSCs are capable of modulating inflammatory (Newman et al., 2009) and immune processes, as well as oxidative stress (Calio et al., 2014), especially under ischemic conditions. Some investigations have shown that MSCs can differentiate into endothelial cells (Pu et al., 2016) and have a protective effect on endothelial cells of cerebral vessels after ischemic stroke (Chung et al., 2015; Liu et al., 2019).
The aim of the study was to elucidate how the intravenous transplantation of MSCs performed on the day of ischemia/reperfusion affected the vascular density, the reactivity of the pial arteries, and tissue perfusion in the cerebral cortex for 7, 14, and 21 days after ischemic exposure.
MATERIALS AND METHODS
The work was carried out on animals from the Collection of Laboratory Mammals of Different Taxonomic Affiliation of the Pavlov Institute of Physiology, Russian Academy of Sciences, supported by the program of bioresource collections of the Federal Agency for Scientific Organizations. The studies were performed in accordance with the regulations established by the Ministry of Health and Social Development of the Russian Federation (no. 708n, August 23, 2010, Laboratory Practice Rules), Directive 2010/63/EU of the European Parliament and the Council of the European Union on the protection of animals used for scientific purposes, as well as recommendations of the Bioethical Commission of the Pavlov Institute of Physiology of the Russian Academy of Sciences.
Animals
Experiments were carried out on male Wistar rats (n = 85). The animals were kept in standard vivarium conditions with natural light and free access to water and food.
Ischemia/Reperfusion
In rats anesthetized with chloral hydrate (intraperitoneally, 43 mg/100 g of body weight), ischemia was induced using the technique of 12-min occlusion of both carotid arteries with simultaneously controlled hypotension (reduction and strict maintenance of blood pressure (BP) at a level of 45 ± 3 mm Hg by collection/reinfusion of blood into a heparinized syringe). Direct measurement of blood pressure was performed through a catheter in the femoral artery connected to a DTXPlusTM sensor (Argon Critical Care Systems, Singapore). It was connected to a computer equipped with original software for the blood pressure imaging developed in our laboratory. At the end of the ischemic period, complete reinfusion of the collected blood was performed. After suturing the surgical wounds and recovering from anesthesia (on heated tables), the animals were returned under conditions of routine maintenance.
MSCs and Their Transplantation
Human mesenchymal stem cells (hMSCs) obtained from a single donor were used for intravenous transplantation. MSC isolation from the bone marrow, their cultivation and phenotyping were carried out at Trans-Technologies LLC according to standard, generally accepted methods with minimal modifications (Azizi et al., 1998; Penfornis, Pochampally, 2011). In particular, culture medium α-MEM (Hyclone, New Zealand) supplemented with 20% fetal bovine serum (Gibco, United States) and 100 μg/mL penicillin/streptomycin (United Staes, United States) were used for hMSC cultivation. Phenotyping of hMSCs was performed by flow cytometry on a FACSscan flow cytometer (Beckton Dickinson, United States). hMSCs were stained with antibodies against positive markers CD90, CD105, CD44, and CD73 and negative markers CD45, CD34, CD14, CD11b, HLA-DR, and 7AAD (Beckton Dickinson, United States). hMSCs at the second and third passages were used for transplantation. Their intravenous transplantation to rats was performed 1 h after I/R of the brain. Each animal was injected with 5x106 cells in 30 μL culture medium. All subsequent surgical and experimental steps were done on rats anesthetized intraperitoneally with 20 mg/kg zoletil (Virbac, France). Euthanasia was performed by introducing an increased dose of zoletil.
Groups of Animals
1. The control group was made up of sham-operated (SO) Wistar rats that underwent surgery but without I/R. Studies of the vascular density, the reactivity of the pial arteries, and perfusion in the sensorimotor cortex in this and all other groups (acute experiments) were carried out 7, 14, and 21 days after surgery. The weight of rats and blood pressure were as follows: after 7 days (n = 9), 245 ± 13.8 g and 139 ± 5.6 mm Hg respectively; after 14 days (n = 10), 303 ± 12.7 g and 133 ± 4.6 mm Hg, respectively; and, after 21 days (n = 9), 330 ± 12.2 g and 135 ± 2 mm Hg, respectively.
2. Wistar rats that had undergone I/R of the brain. Weight and blood pressure after 7 days (n = 9) were 259 ± 12.1 g and 149 ± 4.7 mm Hg, respectively; on the 14th day (n = 8), 256 ± 5.2 g and 133 ± 5.3 mm Hg, respectively; and, after 21 days (n = 9), 318 ± 4.2 g and 124 ± 3.9 mm Hg, respectively.
3. Wistar rats that had undergone I/R of the brain and intravenous injection with hMSCs. Weight and blood pressure after 7 days (n = 9) were 250 ± 11.3 g and 144 ± 4.1 mm Hg, respectively; on the 14th day (n = 10), 306 ± 9.9 g and 134 ± 4.9 mm Hg, respectively; and, on the 21st day (n = 12), 327 ± 6.3 g and 123 ± 5.7 mm Hg, respectively.
Visualization and Monitoring of the Microvascular Network
For in vivo investigation of pial arteries reactions, a hole was drilled in the parietal region of the animal's skull (S ≈ 1 cm2). The dura mater within the hole was removed, thereby opening the area for further investigation. The brain surface was continuously irrigated with Krebs solution (pH 7.4, 37°C), The average blood pressure monitored throughout experiments remained approximately at the same level at 134 ± 5 mm Hg. The body temperature of the animals was maintained at 38°C throughout the experiment. The pial arteries were visualized using an original setup that included an MC-2ZOOM stereoscopic microscope (Micromed, Russia), a color camera, a DCM-510 video eyepiece (Scopetek, China), and a computer. The number of arteries and the total number of microvessels in a certain area were determined on static images using the Photo M software for cytophotometry (created by A. Chernigovsky, http://www.t_lambda.chat.ru). The diameters of the pial arterial vessels were then measured. During the experiment, more than 40 pial arteries were examined in each animal. The diameter of the arteries was fixed under standard conditions with continuous irrigation of the brain surface with Krebs solution and acetylcholine (ACh) solution (10--7 M/L) (Sigma-Aldrich, United States). It has been shown experimentally that pial microvessels of different diameters do not respond to ACh in the same way: the smaller the initial diameter, the greater the response (Gorshkova et al., 2016). In this regard, we also divided all investigated the pial arterial microvessels into groups: more than 40, 20--40, and less than 20 µm. The results of ACh exposure were assessed on the basis of the number of dilated arterial vessels and the degree of their dilation.
In the same experimental animals, perfusion (P) in the cerebral cortex tissue was measured using a LAKK-M multifunctional laser diagnostic complex (LAZMA, Russia). The device sensor was placed at three points above the sensorimotor cortex with approximate coordinates BP = 1, 2, 3 mm from the bregma; SD = 1.0 mm lateral to the sagittal suture. The software attached to the LAKK-M complex automatically calculated the average value of the microcirculation index PI.
Statistical Analysis
The data were assessed using Microsoft Excel 2003 and InStat 3.02 software (GraphPad Software Inc., United States). The results are presented as the mean values and standard errors. Comparison of the mean data of independent samples with a normal distribution in the sample was calculated using analysis of variance followed by pairwise comparison of groups according to Tukey's test. When the variant distribution in the sample differed from the normal, the Kruskal--Wallis test was used, followed by pairwise comparison of the groups according to Dunn's test. p < 0.05 was considered statistically significant.
RESULTS
Flow-cytometry analysis of hMSC culture showed that it consisted 99.7% of CD90+, CD73+, CD105+, and CD44+ cells (MSCs); 0.3% of CD45+ and CD34+ cells (hematopoietic cells); and 0.5% of CD14+, CD11b+, and HLA-DR+. 7AAD+ cells (nonviable) made up no more than 0.9-1%.
Results concerning the density of the microvascular network of the pial membrane of the sensorimotor cortex in SO rats that had undergone I/R of the brain are shown in Fig. 1. After I/R in rats, the density of the entire microvascular network and arterial vessels most significantly decreased compared with SO in the first 7 days---by an average of 1.6 and 1.4 times, respectively. After 14 days, the density of the entire microvascular network and the density of arterial vessels were 1.4 and 1.2 times lower than in the SO group; after 21 days they were 1.2 and 1.3 times lower. In the group of animals that underwent I/R and intravenous transplantation of hMSCs, the density of the microvascular network of the pial membrane remained at the level of the SO rats during the entire study period.
In the group of animals that had undergone I/R of the brain, we revealed significantly declined reactivity of the walls of the pial arteries upon application of ACh to the brain surface (Fig. 2). In rats from this group, the number of arterial vessels that responded to ACh exposure by expanding (increasing in diameter) decreased after 7 days by 1.4--1.7 times; after 14 days, by 1.6--1.9 times, and, after 21 days, by 1.2--1.7 times as compared to SO rats. No differences in diameter changes were found between the groups of animals (data not shown). hMSC transplantation made it possible to preserve the reactivity of the pial arteries at the level of SO animals. It can be seen in Fig. 2 that, in the cell therapy group, the same number of arterial microvessels responded by dilatation to ACh exposure as in the control group. The degree of their dilatation was the same as in the SO animals.
The PI level in the sensorimotor cortex tissue in all animals that underwent I/R of the brain remained approximately the same after 7 and 14 days as in SO rats (Fig. 3). Twenty-one days after I/R, we found a significant decrease in PI (on average, by 1.6 times) in the group of rats exposed to I/R only. In animals of the cell-therapy group, 21 days after ischemia, a decrease in PI was also revealed, but it was less significant (on average, by 1.2 times).
DISCUSSION
Dysfunction of the neurovascular unit in cerebral ischemia is caused by both a drastic decrease in blood flow in the brain tissue and its restoration (Bai, Lyden, 2015; Kalogeris et al., 2017). The optimal therapeutic strategy in the treatment of the consequences of ischemia is to minimize the development of such pathological processes as inflammation, oxidative stress, metabolic disturbances, and endothelial dysfunction. Application of MSCs inhibits tissue inflammation (Newman et al., 2009) and development of oxidative stress (Calio et al., 2014). MSCs secrete neuroprotective substances (Mahmood et al., 2004). In recent years, a number of publications have appeared proving that MSC transplantation restores the function of the endothelium of cerebral vessels (Chung et al., 2015; Abumaree et al., 2017). Earlier, in old, spontaneously hypertensive rats and animals that had undergone nephrectomy, we observed restoration of the density of the microvascular bed of the pial membrane and the function of the endothelium of the pial arteries and PI in the cerebral cortex within a year after intracerebral or intravenous MSC injection (Sokolova, Polyntsev, 2017; Sokolova, Pavlichenko, 2020). We observed the same positive effect from intravenous transplantation of MSCs on the day of ischemic exposure modeling in the presented study. In rats that had undergone I/R after 7, 14, and 21 days, the density of the entire microvascular network of the pial membrane decreased by 1.4, 1.2, and 1.3 times, respectively, and the density of the arterial area decreased by 1.6, 1.4, and 1.2 times, respectively. After cell therapy, the animals completely retained the vascular network of the pial membrane during the entire observation period (Fig. 1). It is likely that, in this case, we are dealing precisely with the preservation of native vessels (especially in the first 7 days) associated with the release of protective factors by MSCs. It is most significant that MSCs not only help to preserve the structure of the microvascular network, but also prevent the development of ED. The ischemic effect drastically negatively affected the state of the endothelial cells of the pial arteries (Fig. 2): 7, 14, and 21 days after I/R of the brain, the number of vessels that responded to ACh application with dilatation was 1.2--1.9 times less than in control rats. We previously demonstrated that, 7 days after ischemia, an increase in the content of circulating endothelial cells was noted in the peripheral blood, which may be associated with enhanced desquamation of endothelial cells as a result of endothelial injury (Gorshkova et al., 2016). The disturbed integrity of the endothelial layer in this postischemic period may be the reason for the subsequent deterioration of vasodilator endothelium-dependent vascular responses to ACh. Consequently, short-term ischemia stimulated ED in pial arterial vessels, and, by 21 days, the endothelial function was not restored. The application of MSCs made it possible to completely prevent the development of ED in ischemic animals during the entire observation period (Fig. 2). Endothelial cells secrete a number of vasoactive substances maintaining homeostasis of the vascular wall and modulating the vascular response to endogenous and exogenous influences (Ierssel et al., 2015). The rate of tissue blood flow is regulated by the relaxation/contraction of smooth-muscle cells in the artery wall. For there to be adequate oxygen supply in the brain tissue, a certain rate of blood flow must be maintained. The perfusion that we measure (PI) is its integral indicator. We observed a statistically significant decrease in PI in all rats that underwent I/R of the brain only on day 21. In the group of animals that received only I/R, the PI decreased by about 1.6 times compared with SO rats. In the cell-therapy group, the decrease in PI was less pronounced---on average, 1.2 times lower than in SO animals---but statistically significantly higher than in rats that underwent only I/R. PI is directly proportional to the speed and number of erythrocytes in the probed tissue volume (Krupatkin, Sidorov, 2013). Retention of the PI level in rats after I/R of the brain in the first 14 days may be explained by increased linear velocity of blood flow and/or with the phenomena of blood stagnation in the vessels of the cerebral cortex. The mechanisms of the decrease in PI after 21 days may include a decrease in the density of the microvascular network; compression of the vascular lumen by swollen processes of astrocytes; and intravascular accumulation of erythrocytes, platelets, and leukocytes (Bai, Lyden, 2015).
We, like other researchers (Kalogeris et al., 2017), connect the positive effect of cell therapy with the paracrine function of MSCs.
In conclusion, intravenous transplantation of hMSCs after ischemia/reperfusion protected the structure of the microvascular network of the pial membrane of the cerebral cortex and prevented the development of endothelial dysfunction in the pial arteries.
FUNDING
This work was carried out with the financial support of the program "Fundamental Scientific Research for Long-Term Development and Ensuring the Competitiveness of Society and the State" (47_110_DRiOK, topic 64.1 (0134-2019-0001) “Discovering the Mechanisms of Interaction of Molecular--Cellular and Systemic Regulation of the Functions of Internal Organs.”
COMPLIANCE WITH ETHICAL STANDARDS
Experiments on rats were carried out in accordance with regulations established by the Ministry of Health and Social Development of the Russian Federation no. 708n of 23.08.10 ("Laboratory Practice Rules"), Directive 2010/63/EU of the European Parliament and the Council of the European Union on the protection of animals used for scientific purposes, and the recommendations of the Bioethics Commission of the Pavlov Institute of Physiology, Russian Academy of Sciences.
CONFLICT OF INTEREST
The authors declare that they have no conflict of interest.
REFERENCES
Abumaree, M., Hakami, M., and Abomaray, F., Human chorionic villous mesenchymal stem/stromal cells modify the effects of oxidative stress on endothelial cell functions, Placenta, 2017, vol. 59, p. 74.
Azizi, S.A., Stokes, D., Augelli, B.J., DiGirolamo, C., and Prockop, D.J., Engraftment and migration of human bone marrow stromal cells implanted in the brains of albino rats—similarities to astrocyte grafts, Proc. Natl. Acad. Sci. U. S. A., 1998, vol. 95, p. 3908.
Bai, J. and Lyden, P., Revisiting cerebral postischemic reperfusion injury: new insights in understanding reperfusion failure, hemorrhage, and edema, Int. J. Stroke, 2015, vol. 10, p. 143.
Calio, M., Marinbo, D., Ko, G., Ribeiro, R., Carbonel, A., Oyama, L., Ormanji, M., Guirao, T., Calio, P., Reis, L., Simoes, M., Lisboa-Nascimento, T., Ferreira, A., and Bertoncini, C., Transplantation of bone marrow mesenchymal stem cells decreases oxidative stress, apoptosis, and hipppocampal damage in brain of a spontaneous stroke model, Free Radical Biol. Med., 2014, vol. 70, p. 141.
Chung, T., Kim, J., and Choi, B., Adipose-derived mesenchymal stem cells reduce neuronal death after transient global cerebral ischemia through prevention of blood-brain barrier disruption and endothelial damage, Stem Cells Trans. Med., 2015, vol. 4, p. 178.
Feng, N., Hao, G., Yang, F., Qu, F., Zheng, H., Liang, S., and Jin, Y., Transplantation of mesenchymal stem cells promotes the functional recovery of the central nervous system following cerebral ischemia by inhibiting myelin-associated inhibitor expression and neural apoptosis, Exp. Ther. Med., 2016, vol. 11, p. 1595.
Fitzsimmons, R., Mazurek, M., Soos, A., and Simmons, C., Mesenchymal stromal/stem cells in regenerative medicine and tissue engineering, Stem Cells Int., 2018, art. 8031718. https://doi.org/10.1155/2018/8031718
Gimbrone, M. and Garcia-Cardena, G., Endothelial cell dysfunction and the pathology of atherosclerosis, Circ. Res., 2016, vol. 118, p. 620.
Gorshkova, O.P., Shuvaeva, V.N., Lensman, M.V., and Artem'eva, A.I., Post-ischemic changes in the vasomotor function of endothelium, Sovrem. Probl. Nauki Obrazov., 2016, vol. 5. URL: http://www.science-education.ru
Ierssel, S., Conraads, V., Craenenbroeck, E., Liu, Y., Maas, A., Parizel, P., Hoymans, V., Vrints, C., and Jorens, P., Endothelial dysfunction in acute brain injury and the development of cerebral ischemia, J. Neurosci. Res., 2015, vol. 93, p. 866.
Kalogeris, T., Baines, C., Krenz, M., and Korthuis, R., Ischemia/reperfusion, Comp. Physiol., 2016, vol. 7, p. 113.
Krupatkin, A.I. and Sidorov, V.V., Funktsional’naya diagnostika sostoyaniya mikrotsirkulyatorno-tkanevykh sistem (Rukovodstvo dlya vrachei) (Functional Diagnostics of State of Microcirculatory-Tissue Systems (Guidelines for Physicians)), Moscow: Librokom, 2013.
Liu, K., Guo, L., Zhou, Z., Pan, M., and Yan, C., Mesenchymal stem cells transfer mitochondria into cerebral microvasculature and promote recovery from ischemic stroke, Microvasc. Res., 2019, vol. 123, p. 74.
Mahmood, A., Lu, D., and Chopp, M., Intravenous administration of marrow stromal cells (MSCs) increases the expression of growth factors in rat brain after traumatic brain injury, J. Neurotrauma, 2004, vol. 21, p. 33.
Manukhina, E.B. and Malyshev, I.Yu., The role of nitrogen oxide in the progression and prevention of endothelial dysfunction, Vestn. VGMU, 2003, vol. 2, no. 2, p. 5.
Newman, R., Yoo, D., and LeRoux, M., Treatment of inflammatory diseases with mesenchymal stem cells, Inflamm. Allergy, 2009, vol. 8, p. 110.
Penfornis, P. and Pochampally, R., Isolation and expansion of mesenchymal stem cells/multipotential stromal cells from human bone marrow, Methods Mol. Biol., 2011, vol. 698, p. 11.
Pu, C., Liu, C., Liang, C., Yen,Y.-H., Chen, S.-H., Jiang-Shieh, Y.-F., Chien, C.-L., Chen, Y.-C., and Chen, Y.-L., Adipose-derived stem cells protect skin flaps against ischemia/reperfusion injury via IL-6 expression, J. Invest. Dermatol., 2016, vol. 137, p. 1353.
Shlyakhto, E.V., Barantsevitch, E.R., Shcherbak, N.S., and Galagudza, M.M., Molecular mechanisms of development of cerebral tolerance to ischemia. Part 1, Vestn. Ross. Akad. Med. Nauk, 2012, vol. 6, p. 42.
Sokolova, I.B. and Pavlichenko, N.N., The efficiency of mesenchymal stem cells in improving microcirculation in the cerebral cortex of rats after nephrectomy, Cell Tissue Biol., 2021, vol. 15, no. 1, p. 12.
Sokolova, I.B. and Polyntsev, D.G., The efficacy of mesenchymal stem cells for the improvement of cerebral microcirculation in spontaneously hypertensive rats, Cell Tissue Biol., 2017, vol. 11, no. 5, p. 343.
FIGURE CAPTIONS
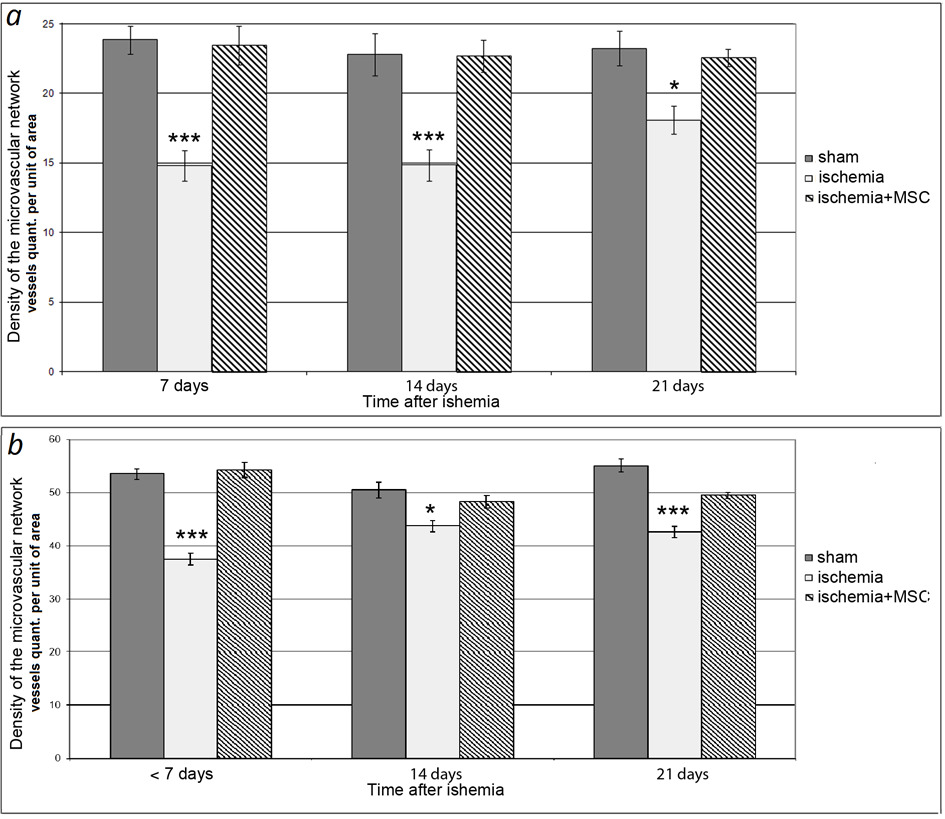
Fig 1. Density of microvascular bed in SO and ischemic rats. (a) Density of arterial section of microvascular network of bone; (b) density of entire investigated section of vascular network. Dark columns, SO rats; light columns, rats with ischemia; hatched columns, rats that underwent ischemia and received intravenous transplantation of hMSCs on the day of surgery. Horizontally, time after ischemia; vertically, density of the microvascular bed. Asterisks show significant difference in comparison with the corresponding values in SO animals of this group (*p < 0.05, ***p < 0.001, Kruskal--Wallis test with subsequent pairwise comparison of groups according to Dunn's test).
KEY:
Время после ишемии—Time after ischemia;
ЛО –SO;
Ишемия-- Ischemia;
Сут—Days;
плотности микрососудистого русла-- density of microvascular bed
количество сосудов на ед площади—vessel number per unit of area
МСКч.--hMSC
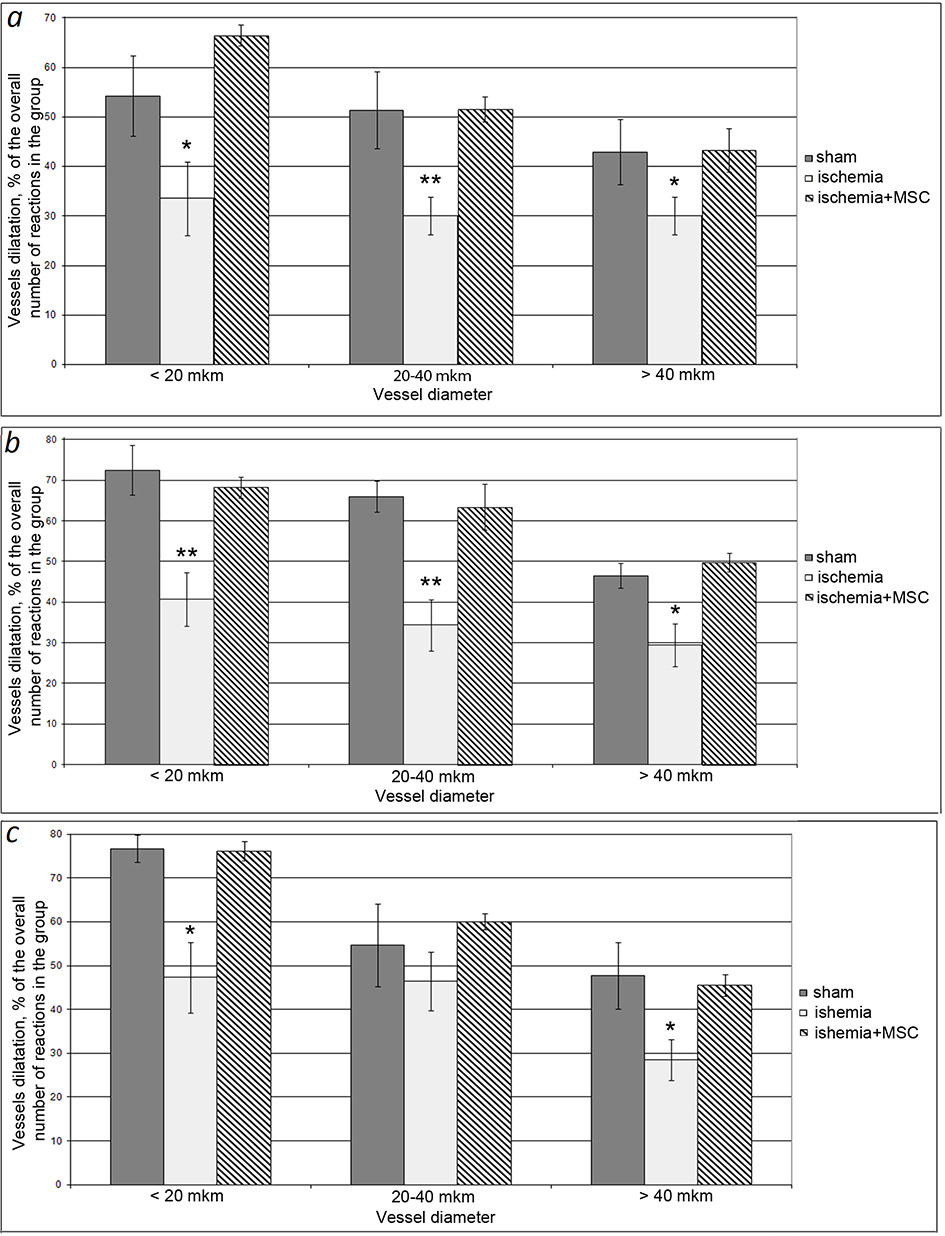
Fig. 2. Number of pial arteries responding with dilatation to acetylcholine exposure. (a) 7 days after ischemia, (b) 14 days after ischemia, (c) 21 days after ischemia. Dark columns, SO rats; light columns, rats with ischemia; hatched columns, rats that underwent ischemia and received intravenous transplantation of hMSCs on the day of surgery. Horizontally, vessel diameter; vertically, number of vessels dilated in response to ACh exposure, % of total number of responses to ACh in the group. Asterisks show significant difference in comparison in comparison with corresponding values in SO animals of this group (*p < 0.05, **p < 0.01, ***p < 0.001, Kruskal--Wallis test with subsequent pairwise comparison of groups according to Dunn's test).
KEY:
диаметр сосудов—vessel diameter;
ЛО –SO;
МСКч.--hMSCs
Ишемия-- Ischemia
Мкм-->µm;
Более—>More;
Менее—> Less;
диляция сосудов, % от общего числа реакций в группе—> Vessel dilation, % of total number of reactions in the group.
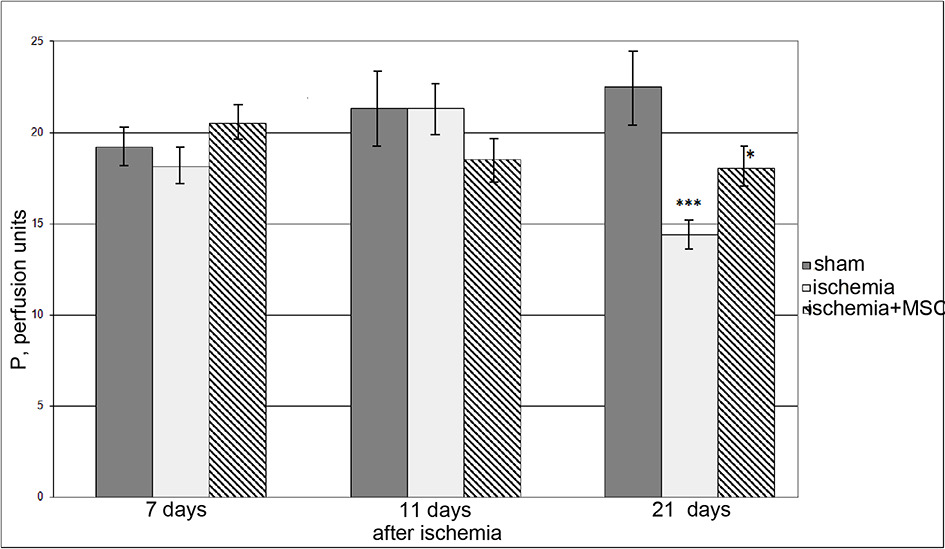
Fig. 3. Changes in the cerebral microcirculation in SO rats and rats with ischemia. Dark columns, SO rats; light columns, rats with ischemia; hatched columns, rats that underwent ischemia and received intravenous transplantation of hMSCs on the day of surgery. Horizontally, time after ischemia; vertically, perfusion index. Asterisks show significant difference in comparison in comparison with corresponding values in SO animals of this group (*p < 0.05, **p < 0.01, ***p < 0.001, Kruskal--Wallis test with subsequent pairwise comparison of groups according to Dunn's test).
KEY:
Время после ишемии—Time after ischemia;
Ишемия-- Ischemia;
МСКч.—hMSC;
Сут—Days;
П. перф. Ед. –P, perfusion units
Translated by I. Fridlyanskaya