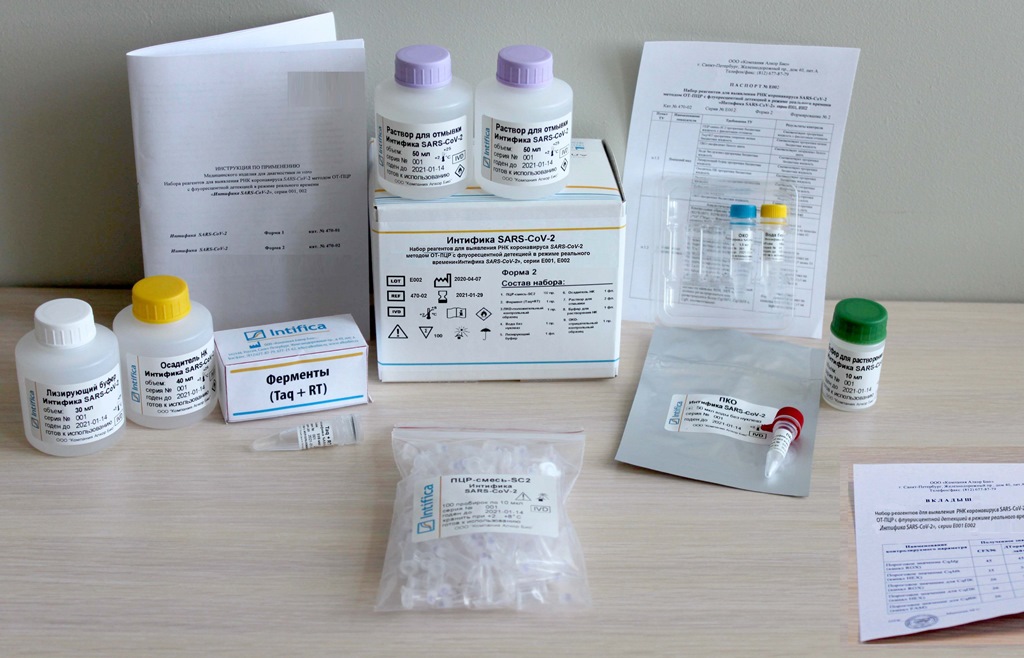
 In the middle of July, Alkor Bio Group of Companies received a registration certificate from Roszdravnadzor for the new kit of reagents "Intifica SARS-CoV-2" for the qualitative detection of SARS-CoV-2 coronavirus RNA by RT-PCR with fluorescence detection in real time.
In the middle of July, Alkor Bio Group of Companies received a registration certificate from Roszdravnadzor for the new kit of reagents "Intifica SARS-CoV-2" for the qualitative detection of SARS-CoV-2 coronavirus RNA by RT-PCR with fluorescence detection in real time.
The kit of reagents "Intifica Sars-CoV-2" has 100% diagnostic sensitivity and specificity, quality control of diagnostics at every stage, including sampling of biomaterial. Alkor Bio Group of Companies released this kit in several configurations, including one where reagents for both the isolation and detection of SARS-CoV-2 RNA are in one kit.
To organize the production of these reagents, repayable financing on preferential terms was provided to the enterprise by the Industrial Development Fund under the program "Counteracting Epidemic Diseases".
The "Intifica Sars-CoV-2" kit is designed for analysis of 100 samples, including controls. Since the genome of the coronavirus continues to mutate, in order to improve the reliability of diagnostics, the detection of SARS-CoV-2 RNA in the kit set of Alkor Bio GCs is carried out using three specific targets (ORF1ab, N, ORF8). This allows the new kit to be used not only as the main one for detecting the SARS-CoV-2 virus, but also as a confirmation one.
 |
 |
 |
 |